
Thioxanthone-Catalyzed Single-Step Synthesis of β- and γ-Amino Acid Derivatives
Contributors: Benjamin List, Margareta M. Poje
Significance
Glorius and co-workers report a thioxanthone-catalyzed iminocarboxylation of alkenes and (hetero)arenes with bifunctional oxime esters. A variety of substrates, ranging from simple alkenes such as ethylene, to heteroaromatic systems, are tolerated under the mild reaction conditions, affording the corresponding β- and γ-amino acid derivatives in modest to excellent yields.
Comment
Experimental and computational studies support a photoinduced triplet–triplet energy transfer mechanism in which N–O bond homolysis leads to the formation of a C-centered ester and N-centered iminyl radical pair. The potential of the method is demonstrated by syntheses of various biologically active molecules.
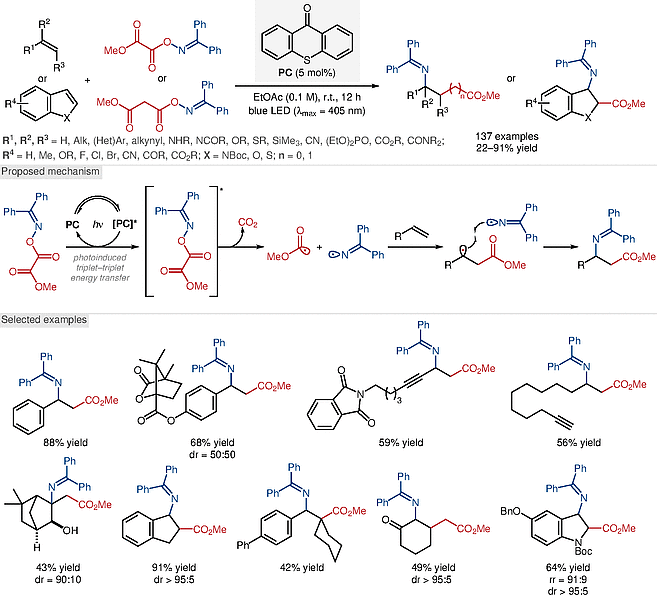
Tan G, Das M, Keum H, Bellotti P, Daniliuc C, Glorius F. * Westfälische Wilhelms-Universität Münster, Germany
Photochemical Single-Step Synthesis of β-Amino Acid Derivatives from Alkenes and (Hetero)Arenes
Nat. Chem. 2022;
14: 1174-1184
DOI: 10.1038/s41557-022-01008-w.
