
The Next Generation Lincosamide: Iboxamycin
Contributor(s): Dirk Trauner, Klaus-Peter Ruehmann
Polikanov YS, *, Myers AG. * et al. University of Illinois at Chicago and Harvard University, Cambridge, USA
A Synthetic Antibiotic Class Overcoming Bacterial Multidrug Resistance
Nature 2021;
599: 507-512
DOI: 10.1038/s41586-021-04045-6.
Mitcheltree MJ, Stevenson JW, Pisipati A, Myers AG. * Harvard University, Cambridge, USA
J. Am. Chem. Soc. 2021;
143: 6829-6835
DOI: 10.1021/jacs.1c03536.
Key words
antibiotics - Sharpless epoxidation - pseudoephenamine auxiliary - ring-closing metathesis - Wacker oxidation
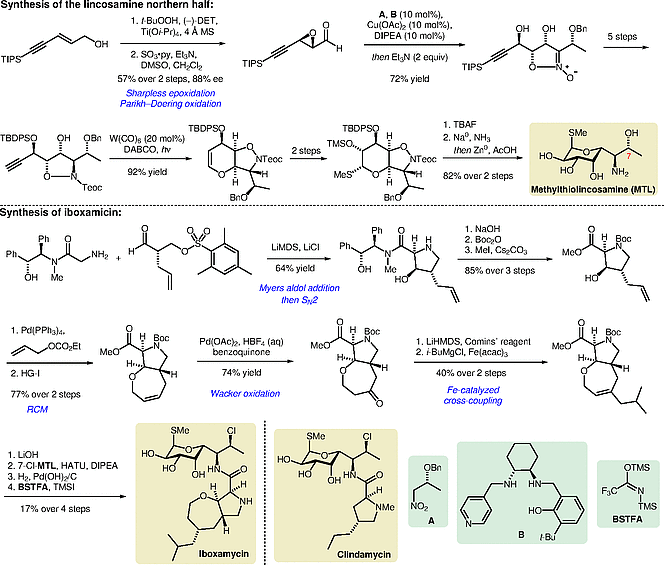
Significance
The lincosamides are a class of antibiotics that acts by inhibition of the bacterial ribosome. 50 years after the semisynthetic derivative clindamycin was approved by the FDA, Myers and co-workers published their component-based total synthetic platform, which resulted in a new, highly potent lincosamide analogue: iboxamycin. It acts in Gram-positive, Gram-negative, and resistant strains.
Comment
The lincosamine northern half was accessed from an epoxy aldehyde and a nitro compound A. The diastereoselective Henry reaction between both building blocks was followed by an epoxide opening that established a cyclic nitronate. Hydroxyproline analogues of the southern half were accessed using the Myers’ pseudoephanamine auxiliary.
